

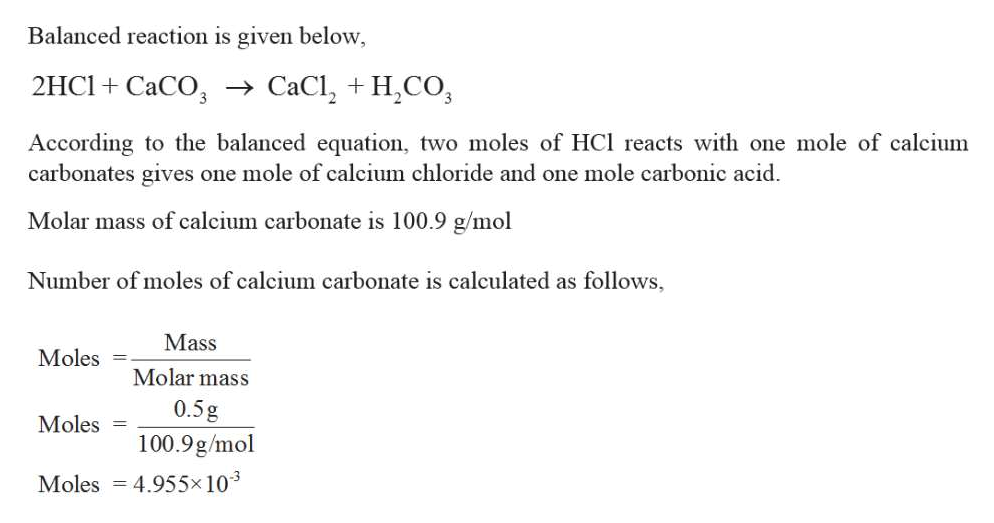
In the sulfation experiments, the gas consisted of 0.5% SO 2 and 3% O 2. All gas concentrations are given in vol% unless otherwise noted. The experiments were performed with varying gas compositions (AGA), but with a total flow fixed at 0.726 L/min. The sulfation experiments with 10 wt % active substance were performed twice to ensure reproducibility of the experimental method. Depending on the amount of the active substance, the total melt height was 4–5 cm. CaO was added for the study of indirect sulfation (10, 27 and 30 wt %) and combined CO 2/SO 2 capture experiments (10 wt %), while CaCO 3 was added for the study of direct sulfation (10 and 30 wt %).
Eutectic CaF 2–CaCl 2 (150–160 g) (13.8 wt % CaF 2 in CaCl 2) was used as reaction media for all experiments. For each experiment, the desired salt composition was prepared by direct mixing of the dried powder salts by means of weighing. (13)Ī summary of the experimental conditions is given in Table 2. However, the practical limit has been found to be 30 wt % because of increased viscosities and slower reactions when the active substances are present substantially above their solubility limits. Furthermore, CO 2 capture has been successfully demonstrated in a melt containing up to 40 wt % CaO. (12) The rapid reaction rates and good utilization of the active substances have been attributed to the high solubility of the formed CaCO 3 in the melt, leaving highly reactive CaO readily available for the incoming CO 2. This was followed by stable sorption capacities throughout the remaining cycles, (10) without any deterioration of the reaction kinetics.
#Cacl2 molar mass series#
A series of 12 cycles has been performed, showing an increased capacity in the first few cycles. (10) In this system, it is possible to utilize up to 85% of the initial CaO during absorption, and all CaCO 3 is completely decomposed during desorption. (9−11) The melt showing most promising absorption and desorption characteristics has been found to be eutectic CaF 2–CaCl 2 (13.8 wt % CaF 2 in CaCl 2). (8) The characteristics of the process have been further investigated experimentally in a one-chamber reactor by bubbling simulated flue gases consisting of CO 2 and N 2 through various chloride and fluoride-based melts. The concept was first reported by Olsen and Tomkute in 2013.
Based on experimental data and thermodynamic modeling, it was estimated that the ratio of activity coefficients for CaO and CaCO 3 is between 5 and 6 when dissolved in eutectic CaF 2–CaCl 2.Ĭarbon capture in molten salts (CCMS) is based on the same principles as CaL but with the active substances (CaO/CaCO 3) dissolved or partly dissolved in molten salts, allowing faster reaction kinetics, higher CO 2 sorption capacity, and avoiding solids attrition issues. This is attributed to higher activities when the active substances are dissolved in molten salts. Furthermore, it was shown that CO 2 capture in molten salts is possible at temperatures above the decomposition temperature for CaCO 3 in solid-state calcium looping. The results indicate that both direct and indirect sulfation occur and that carbon capture is only slightly, but not significantly, inhibited in accordance with the level of SO 2 in the flue gas. The composition of the outlet gas from the reactor was analyzed by Fourier transform infrared spectroscopy. In addition, combined capture experiments with 14 vol % CO 2 and levels of SO 2 up to 0.5 vol % have been performed. Simulated flue gases containing 0.5 vol % SO 2 and 3 vol % O 2 have been used. Experiments have been performed to investigate indirect sulfation of CaO and direct sulfation of CaCO 3 in a eutectic mixture of calcium fluoride (CaF 2) and calcium chloride (CaCl 2) because it is not known whether these are thermodynamically favored in the molten state. In the present work, the focus is on sulfur dioxide (SO 2) and oxygen (O 2), a combination often coexisting with CO 2 in flue gases from power production or industry. The next step in the technology development is to test the performance under more realistic gas compositions. CCMS has previously demonstrated promising properties for carbon capture with simulated flue gases on the laboratory scale. In CCMS, the reversible reaction between calcium oxide (CaO) and carbon dioxide (CO 2) to form calcium carbonate (CaCO 3) is utilized, with the active substances being dissolved or partly dissolved in molten salts. Carbon capture in molten salts (CCMS) is an emerging technology with high potential.

To achieve deep cuts in greenhouse gas emissions from stationary point sources such as carbon intensive industry and fossil power generation, carbon capture and storage (CCS) will be a necessity.


 0 kommentar(er)
0 kommentar(er)
